Tag: Medicine
Tel Aviv University presents an analysis of the reaction of human antibodies to the coronavirus
Patients with severe COVID-19 develop antibodies faster than those with a mild case of the disease.
A diagnostic tool
The researchers found that antibodies of the type IgM, that usually develop at the early stages of viral contagions, developed early in this case only against the protein RBD – the site at which the virus SARS-CoV-2 binds to human cells, and not against the virus’s nuclear protein. “We sampled the antibodies of about 70 COVID-19 patients at the Sharon Hospital, throughout the outbreak of the disease in Israel,” says Prof. Munitz. “Our first finding was that not all viral proteins generate a rapid immune response, but that antibodies targeting the RBD protein did develop very quickly once the symptoms appeared. This finding is quite significant, because it suggests that the test we used may be utilized as a diagnostic tool at different stages of the illness.” “The second thing we noticed, which is even more interesting, is that patients defined as severely ill developed antibodies at a faster rate than mildly ill patients, but ultimately all patients exhibited a similar immune response,” recounts Prof. Munitz. “Patients with mild, moderate and severe COVID-19 all developed the same level of antibodies. This is important, because one might have thought that the severely ill became so sick because they did not develop a sufficient amount of antibodies, and were thus unable to combat the virus effectively. We assume that the fast development of antibodies in these patients indicates that their immune system is hyper-active, but this hypothesis requires further research.”Immunological memory
“We measured the levels of antibodies in the patients’ blood when they arrived at the hospital, during the period of hospitalization and after their release,” explains Prof. Gerlic. “We tried to understand whether the level of antibodies in their blood corresponded in any way to the severity of the illness, whether the antibodies developed in a similar way in all patients, and whether they remained in the blood for long periods of time – a critical factor for the ‘herd immunity’ we all wish to attain. We found that at later stages of the disease, about 50 days after the initial appearance of symptoms, a significant decline occurred in the presence of antibodies types IgM and IgA, regardless of the severity of the illness. In IgG-type antibodies, however, we observed only a slight decrease, even in mildly ill patients. IgG-type antibodies play an extremely important role in the immune response because they can neutralize the protein that binds the virus to human cells to enable contagion – thereby preventing the virus from penetrating the cells. We have not yet examined how the antibody actually works, and we do not know whether or not it neutralizes the virus, but the facts that these antibodies are quickly produced in all patients, and stay in the blood for a long time, suggest that they provide some level of immunity. So far, we have found that IgG-type antibodies remain in the body for two months. We will continue to monitor the patients for another year, to find out how long the antibodies remain in their bodies – hoping for the formation of an immunological memory.” In the new study the researchers from TAU used a new serological test developed in their laboratory. The IDF’s Medical Corps has already used the serological test developed by Prof. Gerlic and Prof. Munitz to detect COVID-19 antibodies in the blood of IDF soldiers. Within the next few weeks the test will be sent to the Israel’s Ministry of Health for validation, so that it may be used in population surveys. “Alongside the interesting findings,” says Prof. Munitz, “we wanted to demonstrate that our method is valid and more effective than the prevalent test for antibodies targeting viral proteins. To this end we examined samples of antibodies from the blood of COVID-19 patients, alongside samples from 200 healthy participants, taken before November 2019. We proved that our test, based on the antibodies, was able to distinguish between those who were ill and those who were not – at very high levels of sensitivity and specificity. One reason for this success is that we screen for three different antibodies: IgM that appears early and declines early, IgA – found on mucous surfaces like the lungs, and IgG, which we intend to test in the long run, because it may possibly lead to immunity.3D printed heart used to test life-saving drugs
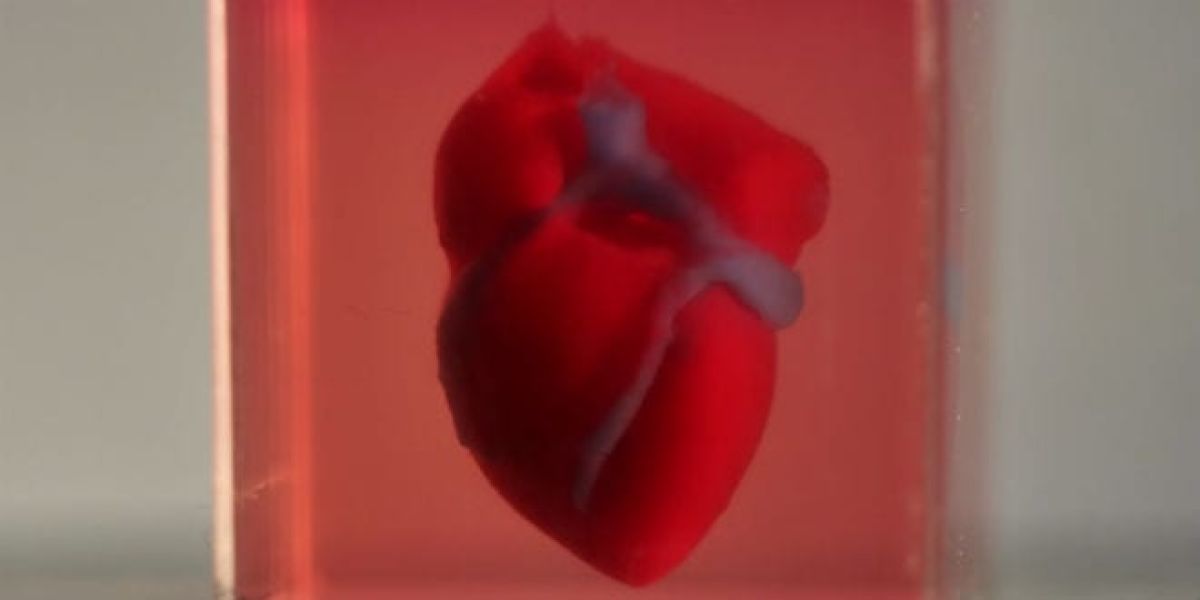
Pharmaceutical company Bayer will test new drugs using human heart tissues 3D-printed in Tel Aviv University
Last April, Prof. Tal Dvir of the George S. Wise Faculty of Life Sciences, the Iby and Aladar Fleischman Faculty of Engineering and his team successfully produced the first-ever 3D-printed heart, from tissue extracted from a patient. The researchers estimate that it will be possible to print personalized organs and tissues within 10-15 years, thus eliminating the need for organ donations and the risk of transplant rejection. Meanwhile, this innovative technology already has the potential to revolutionize a different medical field: drug screening.Saving precious time
“In a Petri dish, all the cells line up in 2D, and it’s only one type of cell” says Prof. Dvir. “In contrast, our engineered tissues are 3D-printed, and therefore better resembles real heart tissues. Our printed tissues contain cardiac muscle, blood vessels and the extracellular matrix which connects the different cells biochemically, mechanically and electrically. Moving away from Petri dishes to 3D printed tissues could significantly improve drug tests, saving precious time and money with the hope of producing safer and more effective medication”. Ramot at Tel Aviv University has signed a collaboration agreement with Bayer to develop and validate a platform for in vitro cardiotoxicity screening, using human heart tissues 3D-printed in Prof. Tal Dvir’s Laboratory for Tissue Engineering and Regenerative Medicine. In upcoming years, Prof. Dvir’s team and Bayer plan to test new medication for toxicity and efficacy using printed whole human hearts. Drug candidates go through several phases of screening before reaching pharmacies. First, the new chemical compound is tested on human tissue cultures. Then, it is administered to lab animals. Finally, the drug is approved for human clinical trials. Prof. Dvir’s 3D-printed tissues could enable faster, cheaper and more efficient screening than Petri dishes. Prof. Dvir hopes to offer Bayer, in the near future, pre-clinical trials on complete printed organs. “Our agreement is just the beginning,” says Prof. Dvir. “Our end goal is to engineer whole human hearts, including all the different chambers, valves, arteries and veins – the best analogue of this complex organ – for an even better toxicological screening process.” To make further use of the application, Ramot at Tel Aviv University licensed the technology to a spin-off company called Matricelf, which first focuses on engineering personalized spinal cord implants to treat paralyzed patients. Matricelf has recently secured a large investment, allowing it to reach clinical settings in the near future.New, innovative drugs
Keren Primor Cohen, Ramot CEO said: “Prof. Dvir’s platform groundbreaking innovation is very promising. We believe that this collaboration with Bayer will support the evaluation and development of new drugs and is a step in building long-term relations with Bayer that we hope will benefit both partners and ultimately patients.” “We are excited to start this new collaboration with Tel Aviv University, which will address a new area of early assessment of safety and tolerability of drug candidates,” said Eckhard von Keutz, Head of Translational Sciences at Bayer. “We already have a global network of partners and this new project will enable Bayer to expand its open innovation activities to Israel, which provides a dynamic ecosystem for innovation in biotech and medical researchDid climate change cause infections 6,000 years ago?
New study of human skulls finds infections peaked due to high population density, poor hygiene and climate conditions
A story in the skulls
Until the advent of antibiotics in the 20th century, ear infections developed into chronic conditions, or, due to complications, caused permanent loss of hearing or even death. “Ear infections are still a very common childhood ailment, with over 50 percent of young children today still suffering from an ear infection at one point or another,” explains Dr. May. “The reason for this is that the tubes that channel fluid from the middle ear to the mouth are underdeveloped in young children, so fluids that accumulate in the ear ultimately cause inflammation.” “A prolonged ear infection would cause permanent damage to the bony wall of the middle ear, which is remarkably preserved into adulthood, so when we sought to investigate changes in communal health over time in our region, we chose to focus on ear infections, developing a special method for doing so,” she adds. The scientists used a videoscope, a tiny camera mounted at the end of a flexible tube, which they inserted through the ear canal to the middle ear to observe its bony walls. In addition, they scanned skull remains with a high-resolution micro-CT, and also examined the middle ear’s bony wall using a light microscope.More room, fewer infections
As living conditions improved, morbidity as a result of ear infections dropped, according to the study. “Houses were larger and featured several rooms, including separate areas for specific activities, i.e. the kitchen was set up in a separate room or outside, and livestock were kept in a separate area,” she says. “The change in lifestyle and climate is reflected in a decline in morbidity.” “Our study deals with the impact of the environment and social behavior on morbidity rates, and to do so, we examined a common disease that has accompanied humanity since inception – the ear infection,” concludes Dr. May. “Understanding how diseases appear, spread and disappear throughout human history can help prevent and find solutions to contemporary illnesses. The study clearly points out risk factors and shows how lifestyle changes can affect the incidence of the disease. In both ear infections and COVID-19, social distancing and adherence to hygiene reduced the spread of infection, while close quarters and unhygienic living conditions saw infections spike.”New TAU study tracks coronavirus spread patterns in Israel
Research finds approximately 70% of the infections in Israel were caused by a SARS-CoV2 strain imported from the United States
The origins of coronavirus
“The novel coronavirus is characterized by mutations that occur at a set pace,” explains Dr. Stern. “These mutations do not affect the virus, i.e. it remains stable, but these mutations can help us trace the chain of infection from country to country. After the pandemic broke out in Wuhan, for example, one or two mutations occurred, and one virus with a mutation may have migrated to Europe where it experienced additional mutations, and from there it traveled to the United States, and so on. “We can look at these mutations as a kind of barcode that helps us keep track of the progression and transformation of the coronavirus as it moves from country to country.” To obtain a clear picture of the origin of infection in Israel, the researchers compared the genomic sequences of local patients to some 4,700 genomic sequences taken from patients around the world. They found that more than 70% of the patients had been infected by a coronavirus strain that originated in the U.S. The remaining nearly 30% of infections were imported from Europe and elsewhere: Belgium (8%), France (6%), England (5%), Spain (3%), Italy (2%), the Philippines (2%), Australia (2%) and Russia (2%). According to Dr. Stern, the new genomic map provides insight into the precise spread of the novel coronavirus within Israel. Until now, any assessment of the spread of infection relied on such subjective parameters as patient feedback. The new research will be able to expose the rate of infection in a household, in an apartment building, in a school, in a neighborhood, and more. It will also provide early detection of super spreaders – people who travel far and wide and infect a large number of people – and could even identify major events with the potential to trigger widespread infection.The importance of 10%
“Going forward, the data obtained from genomic sequencing will serve as an important basis for informed decisions about which institutions to close, for what amount of time, and in which format,” says Dr. Stern. With policymakers in mind, the researchers developed a complex statistical model based on genomic sequencing that estimates the epidemiological parameters of viral spread. The model shows that the rate of infection decreased significantly following strict quarantine measures taken in Israel and highlights a major discrepancy between the number of people each coronavirus patient infected. The model also estimates that over 80% of coronavirus cases in Israel were the direct result of only 10% of the coronavirus patients in Israel, meaning that these 10% were, in fact, super-spreaders. According to the model and to the genomic sequencing, Dr. Stern says that no more than 1% of the population in Israel contracted the virus – a far cry from herd immunity. “In our study, we performed the first massive genomic sequencing of the coronavirus in Israel,” she concludes. “This technology and the information it provides is of great importance for understanding the virus and its spread in the population, as a scientific and objective basis for local and national decision-making. The data obtained from the research can greatly help policymakers on issues such as closures and quarantines. In doing so, the study makes a significant contribution to dealing with the epidemic in Israel, and, more importantly: We have developed tools that will allow us to cope, in real time, with the next outbreak that may occur.”Accurate 3D imaging could significantly improve IVF treatments
New Tel Aviv University technology allows clinicians to identify and select better-quality sperm, potentially increasing chances of pregnancy
Choosing the right cells to make a baby
Under natural fertilization in the woman’s body, the fastest sperm to reach an egg is supposed to bear high-quality genetic material. Progressive movement allows this “best” sperm to overcome the veritable obstacle course of a woman’s reproductive system. “But this ‘natural selection’ is not available to the embryologist, who selects a sperm and injects it into the egg,” Prof. Shaked says. “Sperm cells not only move fast, they are also mostly transparent under regular light microscopy, and cell staining is not allowed in human IVF. Existing imaging technology that can examine the quality of the sperm’s genetic material may cause embryonic damage, so that too is prohibited. In the absence of more precise criteria, sperm cells are selected primarily according to external characteristics and their motility while swimming in water in a dish, which is very different from the natural environment of a woman’s body. “In our study, we sought to develop an entirely new type of imaging technology that would provide as much information as possible about individual sperm cells, does not require cell staining to enhance contrast, and has the potential for enabling the selection of optimal sperm in fertilization treatments.”A hologram of sperm cells
The researchers chose light computed tomography (CT) technology for the unique task of sperm cell imaging. “In a standard medical CT scan, the device rotates around the subject and sends out X-rays that produce multiple projections, ultimately creating a 3D image of the body,” says Prof. Shaked. “In the case of the sperm, instead of rotating the device around this tiny subject, we relied on a natural feature of the sperm itself: Its head is constantly rotating during the forward movement. We used weak light (and not X-rays), which does not damage the cell. We recorded a hologram of the sperm cell during ultrafast movement and identified various internal components according to their refractive index. This creates an accurate, highly dynamic 3D map of its contents without using cell staining.” Using this technique, the researchers obtained a clear and accurate CT image of the sperm at very high resolution in four dimensions: three dimensions in the space at resolution of less than half a micron (one micron equals one millionth of a meter) and the exact time (motion) dimension of the second sub-millisecond. “Our new development provides a comprehensive solution to many known problems of sperm imaging,” Prof. Shaked says. “We were able to create high-resolution imaging of the sperm head while it was moving fast, without the need for stains that could harm the embryo. The new technology can greatly improve the selection of sperm cells in vitro, potentially increasing the chance of pregnancy and the birth of a healthy baby. “To help diagnose male fertility problems, we intend to use our new technique to shed light on the relationship between the 3D movement, structure and contents of sperm and its ability to fertilize an egg and produce a viable pregnancy,” Prof. Shaked concludes. “We believe that such imaging capabilities will contribute to other medical applications, such as developing efficient biomimetic micro-robots to carry drugs within the body.”TAU partners with pharma company to develop COVID-19 vaccine
The epitope-based vaccine will target the most vulnerable part of the viral spike protein
Targeting the Achilles’ heel of coronavirus
“The smaller the target and the focus of the attack, the safer and greater the effectiveness of the vaccine,” he adds. “The virus takes far-reaching measures to hide its RBM from the human immune system, but the best way to ‘win the war’ is to develop a vaccine that specifically targets the virus’s RBM.” Keren Primor Cohen, Ramot CEO says: “We hope that through this collaboration with Neovii, it will be possible to produce an effective vaccine that targets the coronavirus’s Achilles’ heel and will accelerate the development of a protective vaccine against this global threat.” Jürgen Pohle, Neovii CEO, adds: “The outbreak of the COVID-19 pandemic has demonstrated how fragile and vulnerable our societies are in the face of a pandemic. We are extremely excited about our collaboration with Professor Gershoni and TAU which provides Neovii with a first-in-class platform for the rapid development of promising vaccine candidates towards any future emerging pandemics including COVID-19. Furthermore, the COVID-19 vaccine is highly synergistic to Neovii’s core expertise in the development and manufacturing of passive polyclonal antibodies and provides an opportunity to bring a COVID-19 immunotherapy in a rapid manner.” Neovii’s long-standing and well-established experience and capabilities in developing, manufacturing and commercializing biopharmaceuticals will support the objective to have a vaccine ready for use in the general population on an accelerated timeline.What about the environment?
In an online seminar, Prof. Colin Price connects the dots between two current disasters: the climate crisis and the coronavirus crisis
The coronavirus pandemic caught us off guard. Without any warning, it transformed our daily routines overnight and caused a global shock, which will take a long time to recover from. The climate crisis, on the other hand, has been a subject of discussion for scientists for many years. Are there any points at which these two threats connect? And will dealing with the immediate threat, the coronavirus, also lead to more ecological and considerate behavior towards the environment? Prof. Colin Price, head of the Porter School of Environmental Studies and Earth Sciences, created an online seminar on the topic and called on heads of state to take advantage of the opportunity we’ve been given to save the world from an irreversible crisis which is about to reach the point of no return.
Immediate sacrifice for a long-term solution
Since the beginning of the coronavirus crisis, we’ve realized in a few weeks how much power nature has, and how it can neutralize human systems. Entire populations and sectors have been shut down and defined as high risk, economies and medical systems have been paralyzed, prosperous industries are suddenly at the brink of collapse, disrupted supply chains and more.
“Everyone understands that we have to act swiftly around the coronavirus, in order to achieve a fair, long-term solution,” says Prof. Price, and immediately points out the similarity to an issue we’ve been dealing with for a long time – the climate crisis. “While the Corona crisis is mostly threatening to humans, the effects of the climate crisis, evident in deadly heat waves, huge fires, floods, storms and sea level rise, are hitting a much wider ‘target audience’. Animals, infrastructure, natural resources – everything is under threat. The main problem is that because the climate crisis is not immediate, like coronavirus, and because it’s not personal – it’s not taken seriously enough, and long-term solutions aren’t being considered.”
Professor Price referred to similar points in both crises, including the weight that must be given to the exponential curve of both, the speed with which we have to react in order to stop the destructive effects in time, and the immediate sacrifice that must be made to gain a better long-term outcome for future generations.
Change will be good for us and the environment
“There is no doubt that when we go back to normal after the coronavirus crisis, everything will be different,” says Prof. Price. “I estimate that thanks to online communication, we will see a decrease in the volume of business trips abroad and, as a result, the demand for oil will decrease. People will work more from home and there will no doubt be companies that adopt this format, and will completely give up paying office fees. There will be greater investment in new infrastructure in health systems, distance learning, local manufacturing and more changes that, if examined in the environmental aspect – are undoubtedly friendly to the natural resources we breathe and consume, which we want to protect.”
“If you ask me what we should learn from the current crisis, it’s first and foremost that we should listen to scientists. From the first day the virus broke out in China, they warned that it was an epidemic that would affect the whole world. So should we listen to the scientists who have been studying the climate crisis. I’ve been working in the field for 30 years and I can say for sure – people don’t listen. World leaders don’t listen.”
“We all need to understand the severity of the exponential curve, and understand the cost of our actions for the global village we live in, both on the coronavirus and climate issues. We have to remember that our actions on one side of the world affect people and the environment on the other.”
Lower the level of vulnerability and increase our resilience
According to Prof. Price, the role of governments is critical in managing the world response to both crises. “To reinvigorate the economy at the end of the Corona crisis, we have the opportunity to encourage funding, job allocation and investment in green technologies, for example in the public transport sector, to make it more efficient, cost-effective and green, to provide a more promising future and prevent the arrival of the next crisis.”
“The risk of a crisis, no matter if it’s an earthquake, fire or deadly virus, depends on three main variables: the threat itself, who is exposed to it and what level of vulnerability there is,” Prof. Price explains. “Once we manage to neutralize one of these variables, our chances of getting hurt are smaller.”

Prof. Colin Price