Tag: Medicine
An Experimental Drug for Alzheimer’s May Help Children with Autism
Tel Aviv University Researchers Discover Alzheimer’s-Like Traits in Autistic Child’s Brain
An extensive international study led by Tel Aviv University, headed by Prof. Illana Gozes of the Department of Human Molecular Genetics and Biochemistry, found deposits of the tau protein typically found in Alzheimer’s patients in tissues taken from the postmortem brain of a 7-year-old autistic child. The child suffered from the ADNP syndrome, an ADNP mutation that causes a deficiency/malfunctioning of the ADNP protein which is essential for brain development. The ADNP syndrome child was characterized by severe developmental delay, intellectual disability, and autism. In light of these findings, the researchers tested an experimental drug called NAP – originally developed for Alzheimer’s disease – on nerve cells in a model of ADNP syndrome with the mutation inducing Alzheimer’s-like symptoms. The experiment was a success, with the damaged nerve – like cells returning to normal function.
The study was conducted in close collaboration with researchers from the Blavatnik School of Computer Science at Tel Aviv University, Sheba Medical Center, and a variety of research institutions across Europe, including the biotechnology institute BIOCEV in the Czech Republic, the Aristotle University of Thessaloniki in Greece, the University of Antwerp in Belgium, and the University Hospital Centre in Zagreb, Croatia. The article was published in July 2020 in the journal Translational Psychiatry printed by the Nature Publishing Group.
Prof. Gozes explains that the current study is based on tissues taken from the brain of a 7-year-old boy with ADNP syndrome who died in Croatia. “When we compared the postmortem ADNP syndrome brain tissues to tissue from the brain of a young person without ADNP syndrome, we found deposits of the tau protein in the ADNP child, a pathology that characterizes Alzheimer’s disease.”
The researchers then “treated” damaged nerve-like cells carrying an ADNP mutation, similar to the deceased child mutation with a drug candidate called NAP, which is developed in Prof. Gozes’s laboratory and originally intended to be used to help treat Alzheimer’s disease. “NAP is actually a short active fragment of the normal ADNP protein,” says Prof. Gozes. “When we added NAP to the nerve cells carrying an ADNP mutation, the tau protein bound to the nerve cell skeleton properly, and the cells returned to normal function.”
Prof. Gozes: “The fact that NAP treatment has been successful in restoring the normal function of neuronal-like cell models with impaired ADNP raises hopes that it may be used as a remedy for ADNP syndrome and its severe implications, including autism. Moreover, because other genetic disorders related to autism are characterized by tau pathologies in the brain, we hope that those suffering from these syndromes will also be able to benefit from NAP treatment in the future.” It is important to note that NAP (also called CP201) has been classified as an “orphan drug” by the US Food and Drug Administration, and is currently in the preparatory stages of a clinical trial in children with ADNP syndrome through the company Coronis Neurosciences.
In another phase of the study, the researchers sought to broaden their understanding of the effects of the mutation that causes ADNP syndrome. To do this, they extracted the genetic material mRNA (messenger RNA) from the tissues of the deceased child, and performed an expression analysis of about 40 proteins in the same child, encoded by the mRNA. Full genetic sequencing was also performed to determine protein expression in white blood cells taken from three other children with ADNP syndrome. An in-depth study was carried out on all of the data obtained in the genetic sequencing using advanced bioinformatics computational tools. The data were compared to online databases of protein expression data from healthy individuals, revealing a variety of characteristics that were common to the children with the syndrome, but very different from the normal appearance of these proteins.
Prof. Gozes concludes that “the significance of these findings is that the mutation that causes ADNP syndrome damages a wide range of essential proteins, some of which bind to, among other things, the tau protein, and impair its function as well. This creates various pathological effects in the brains (and other tissues) of children with ADNP syndrome, one of which is the formation of tau deposits, known to be a characteristic of Alzheimer’s disease. The vast and in-depth knowledge we have accumulated through the present study opens the door to further extensive and diverse research. We hope and believe that we will ultimately reach the goal of developing a drug or drugs that will help children with autism resulting from genetic mutations.”
Featured image: Prof. Illana Gozes
The window of opportunity for preventing metastases
Immune System Stimulating Treatment to Reduce Psychological and Physiological Stress Prevents Metastases and Can Save Cancer Patients’ Lives
In a breakthrough research published recently in Nature magazine, Tel Aviv University researchers found that the short time period around the tumor removal surgery (the weeks before and after surgery), is critical for prevention of metastases development, which develop when the body is under stress.
According to the researchers, to prevent development of metastases after the surgery, patients need immunotherapeutic treatment along with treatment to reduce inflammation, and physical and psychological stress. The research was conducted by Prof. Shamgar Ben-Eliyahu from the School of Psychological Sciences and Sagol School of Neuroscience at Tel Aviv University and Prof. Oded Zmora from Assaf Harofe Medical Center.
Immunotherapeutic treatment is a medical treatment activating the immune system. One such treatment, for example, is injecting substances with similar receptors to those of viruses and bacteria into the patient’s body. The immune system recognizes them as a threat and activates itself, thus it can prevent a metastatic disease.
Prof. Ben-Eliyahu explains: Surgery for the removal of the primary tumor is a mainstay in cancer treatment, however the risk of developing metastases after surgery is estimated at 10% among breast cancer patients, at 20%-40% among colorectal cancer patients, and at 80% among pancreas cancer patients.
According to Prof. Ben-Eliyahu, when the body is under physiological or psychological stress, such as a surgery, groups of hormones called prostaglandin and catecholamine are being produced in large quantities. These hormones suppress the immune system cells’ activity, and thus indirectly increase the development of metastases. Additionally, these hormones help tumor cells left after the surgery to develop into life threatening metastases. Thus, exposure to those hormones cause tumor tissues to become more aggressive and metastatic.
“Medical and immunotherapeutic intervention to reduce psychological and physiological stress, and activate the immune system in the critical period before and after the surgery, can prevent development of metastases, which will be discovered months of years later,” stresses Prof. Ben-Eliyahu.
Prof. Ben-Eliyahu adds that anti-metastatic treatment today skips the critical period around the surgery, thus leaves the medical staff to face the consequences of treating progressive and resistant metastatic processes, which are much harder to stop. Prof. Ben-Eliyahu’s research contradicts the assumption, widespread in the medical community, according to which, just like in chemo and radiotherapy, it is not recommended to give cancer patients immunotherapeutic treatment in the month before and after the surgery.
TAU Researcher Fights Epidemics Both Viral and Virtual
Dan Yamin can detect any kind of contagious outbreak
Think viral, tweet viral
Before joining TAU, Yamin completed a post-doctoral fellowship at Yale University’s School of Public Health. While there, he was disturbed by the level of anti-Israel sentiment on American social networks and its ability to go viral. He immediately made the connection. No paragraph breakBased on the same patterns he studied in disease transmission, Yamin began creating a system that uses artificial intelligence to identify how certain groups use viral marketing tactics to spread anti-Semitic and anti-Israel messages. The system, known as Iron Dome for Social Media, aims to track and identify malicious content with potential to go viral in social media terms. Yamin explains that people who retweet posts casually are much like asymptomatic disease carriers. Many Twitter users will pass on information with covert or explicit anti-Semitic messages unintentionally. Choosing when to respond on social media is a delicate matter. Hence, Yamin suggests using AI, such as in his Iron Dome system, to assist with the decision-making process. “Being proactively pro-Israel on social media is not always the best approach,” says Dr. Yamin. “Most anti-Israel tweets are not viral, so why waste time on tweets that won’t go anywhere?”The next generation of disease control
As Israel and the world face the second wave of COVID-19, Yamin says, “for the time being, we need to live with this virus. If we act responsibly and maintain the daily routine for the vast majority of the population, we will not reach catastrophe”. Looking ahead, Yamin believes data-based methodologies like his are crucial in managing future viral diseases. As such, Yamin will be a key member of TAU’s multidisciplinary Center for Combating Pandemics, the first center of its kind in the world. “Data systems such as this one can substantially improve the accuracy of medical diagnosis in the future,” he says. Dr. Dan Yamin (Photo: Moshe Bedarshi)TAU study: Oxygen therapy improves cognitive function in seniors
Research Published in Aging first to Show Enhanced Brain Function and Cognitive Capabilities Resulting from Novel Therapy
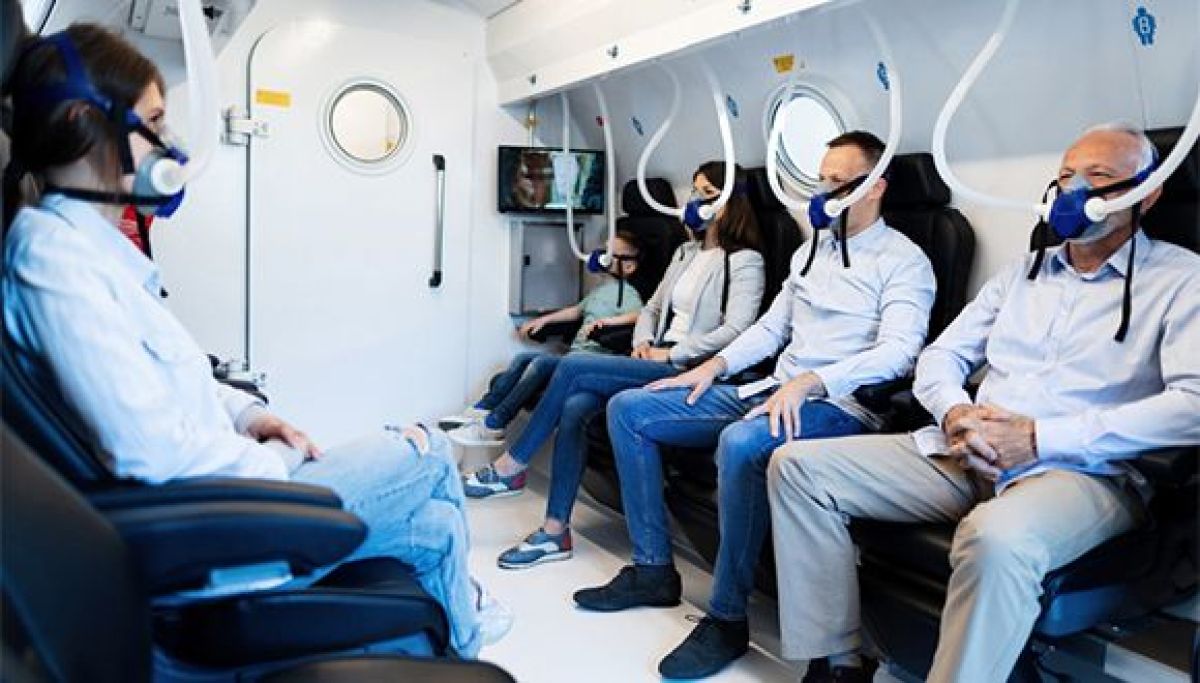
The Sagol Center for Hyperbaric Medicine and Research at Shamir Medical Center, together with Sackler School of Medicine and Sagol School of Neuroscience at Tel Aviv University announced that, for the first time, in humans, a peer-reviewed study has demonstrated that hyperbaric oxygen therapy (HBOT) can significantly enhance the cognitive performance of healthy older adults. The main areas of improvement were attention, information processing speed, executive function, in addition to the global cognitive function, all of which typically decline with age. Moreover, there was a significant correlation between the cognitive changes and improved cerebral blood flow in specific brain locations. The study was published on July 15th, 2020 in the peer reviewed journal Aging, entitled: Cognitive enhancement of healthy older adults using hyperbaric oxygen: a randomized controlled trial. Professor Shai Efrati, Head of the Sagol Center for Hyperbaric Medicine and Research, and Head of Research & Development at Shamir Medical Center, and an Associate Professor at Sackler School of Medicine and Sagol School of Neuroscience at Tel Aviv University, and Dr. Amir Hadanny, the Sagol Center for Hyperbaric Medicine and Research, designed the study based on a unique HBOT protocol developed at the Sagol Center over the past 10 years. The randomized controlled clinical trial included 63 healthy adults (>64) who underwent either HBOT (n=33) or a control period (n=30) for three months. The study’s primary endpoint included a change in general cognitive function measured by a standardized comprehensive battery of computerized cognitive assessments before and after the intervention or control. Cerebral blood flow (CBF) was evaluated by a novel magnetic resonance imaging technique for brain perfusion. “Age-related cognitive and functional decline has become a significant concern in the Western world. Major research efforts around the world are focused on improving the cognitive performance of the so-called ‘normal’ aging population,” said Prof. Efrati. “In our study, for the first time in humans, we have found an effective and safe medical intervention that can address this unwanted consequence of our age-related deterioration.” “Over years of research, we have developed an advanced understanding of HBOT’s ability to restore brain function. In the past, we have demonstrated HBOT’s potential to improve/treat brain injuries such as stroke, traumatic brain injury and anoxic brain injury (due to sustained lack of oxygen supply) by increasing brain blood flow and metabolism,” explained Dr. Amir Hadanny. “This landmark research could have a far-reaching impact on the way we view the aging process and the ability to treat its symptoms.” During HBOT, the patient breaths in pure oxygen in a pressurized chamber where the air pressure is increased to twice that of normal air. This process increases oxygen solubility in the blood that travels throughout the body. The added oxygen stimulates the release of growth factors and stem cells, which promote healing. HBOT has been applied worldwide mostly to treat chronic non-healing wounds. There is a growing body of evidence on the regenerative effects of HBOT. The researchers have demonstrated that the combined action of delivering high levels of oxygen (hyperoxia) and pressure (hyperbaric environment), leads to significant improvement in tissue oxygenation while targeting both oxygen and pressure sensitive genes, resulting in restored and enhanced tissue metabolism. Moreover, these targeted genes induce stem cell proliferation, reduce inflammation and induce generation of new blood vessels and tissue repair mechanisms. “The occlusion of small blood vessels, similar to the occlusions which may develop in the pipes of an ‘aging’ home, is a dominant element in the human aging process. This led us to speculate that HBOT may affect brain performance of the aging population,” Prof. Efrati explained. “We found that HBOT induced a significant increase in brain blood flow, which correlated with cognitive improvement, confirming our theory. One can conjecture that similar beneficial effect of HBOT can be induced in other organs of the aging body. These will be investigated in our upcoming research.” The research group leader, Professor Shai Efrati, who serves as director of The Sagol Center for Hyperbaric Medicine and Research, and is an Associate Professor at Sackler School of Medicine and Sagol School of Neuroscience at Tel Aviv University, also disclosed his role with Aviv Scientific LTD, which has developed a comprehensive program that includes HBOT treatment, cognitive and physical training and nutritional coaching, to enhance brain and body performance of aging adults based on the Sagol HBOT protocol at Aviv Clinics. Prof. Efrati serves as Chair of Aviv Scientific’s Medical Advisory BoardTAU-led Team Destroys Cancer Cells with Ultrasound
Breakthrough method may be applicable to Parkinson’s, Alzheimer’s and more
An international research team, headed by Dr. Tali Ilovitsh from the Biomedical Engineering Department at Tel Aviv University, developed a noninvasive technology platform for gene delivery into cancer cells (breast cancer). The technique combines ultrasound together with tumor-targeted microbubbles. Once the ultrasound is activated, the microbubbles explode like smart and targeted warheads, creating holes in cancer cells’ membranes, and enabling the gene delivery. The two-year research was recently published in the prestigious journal Proceedings of the National Academy of Sciences (PNAS).
Dr. Ilovitsh developed this breakthrough technology during her post doctorate research at the lab of Prof. Katherine Ferrara at Stanford University. The technique utilizes low frequency ultrasound (250 kHz) to detonate microscopic tumor-targeted bubbles. In vivo, cell destruction reached 80% of tumor cells.
Dr. Ilovitsh explains: “Microbubbles are microscopic bubbles filled with gas, with a diameter as small as one tenth of a blood vessel. At certain frequencies and pressures, sound waves cause the microbubbles to act like balloons: they expand and contract periodically. This process increases the transfer of substances from the blood vessels into the surrounding tissue. We discovered that using lower frequencies than those applied previously, microbubbles can significantly expand, until they explode violently. We realized that this discovery could be used as a platform for cancer treatment and started to inject microbubbles into tumors directly.”
Dr. Ilovitsh and the rest of the team used tumor-targeted microbubbles, that were attached to tumor cells’ membranes at the moment of the explosion, and injected them directly into tumors in a mouse model. “About 80% of tumor cells were destroyed in the explosion, which was positive on its own,” says Dr. Ilovitsh. “The targeted treatment, which is safe and cost effective, was able to destroy most of the tumor. However, it is not enough. In order to prevent the remaining cancer cells to spread, we needed to destroy all of the tumor cells. That is why we injected an immunotherapy gene alongside the microbubbles, which acts as a Trojan horse, and signaled the immune system to attack the cancer cell.”
On its own, the gene cannot enter into the cancer cells. However, this gene aimed to enhance the immune system was co-injected together with the microbubbles. Membrane pores were formed in the remaining 20% of the cancer cells that survived the initial explosion, allowing the entry of the gene into the cells. This triggered an immune response that destroyed the cancer cell.
“The majority of cancer cells were destroyed by the explosion, and the remaining cells consumed the immunotherapy gene through the holes that were created in their membranes. The gene caused the cells to produce a substance that triggered the immune system to attack the cancer cell. In fact, our mice had tumors on both sides of their bodies. Despite the fact that we conducted the treatment only on one side, the immune system attacked the distant side as well.”
Potential treatment for brain diseases such as Parkinson’s and Alzheimer’s
Dr. Ilovitsh says that in the future she intends to attempt using this technology as a noninvasive treatment for brain related diseases such as brain tumors and other neurodegenerative conditions such as Alzheimer’s and Parkinson’s disease. “The Blood-Brain barrier does not allow for medications to penetrate through, but microbubbles can temporarily open the barrier, enabling the arrival of the treatment to the target area without the need for an invasive surgical intervention.”
Photo: Dr. Tali Ilovitsh